Cancer Therapeutics and Screening of Anti-Cancer Drugs
Cancers of Interest
- Breast Cancer
- Neuroblastomas
- Colorectal Cancer
- Prostate Cancer
Mechanisms and Pathways of Interest
- AMPK signaling
- Angiogenesis
- Autophagy
- Ca2+ signaling mechanisms
- Endoplasmic Reticulum Stress
- Epithelial-Mesenchymal Transition (EMT)
- mTOR signaling
- Multi-drug resistance
- Other key pathways/mechanisms
Natural substances as potential anti-cancer drugs
Diabetes and Cancer
Diabetes and breast cancer (Triple Negative Breast Cancers; TNBCs)
Type 2 diabetes mellitus (hereafter termed diabetes) is one of the most common chronic diseases worldwide and affects all groups in society, including children, women, youth and adults. Evidence also links a higher incidence of cancer and cancer progression to diabetes. Among the different forms of cancer, studies have reinforced a link between breast cancer risk and diabetes. It has also been reported that postmenopausal women 50 years or older who have diabetes have about a 20-27% increased risk of breast cancer (BC). Furthermore, diabetic breast cancer subjects have as much as a 50% increased chance of mortality when compared to their non-diabetic counterparts. Although several meta-analysis studies have supported the link between diabetes, incidence of breast cancer and mortality among breast cancer subjects, the molecular mechanism(s) behind this link remains elusive.
Among the different forms of breast cancer, triple-negative breast cancers (TNBCs) do not benefit from hormonal or HER2 targeted therapies since they lack estrogen receptors (ER), progesterone receptors (PR) and human epidermal growth factor receptor 2 (HER2). Currently, alternative targeted therapies are lacking, and chemotherapy remains the primary systemic treatment for patients with TNBC. Due to its aggressive nature, early recurrence, and rapid metastasis, a major effort to identify potential molecular targets to treat patients who present with these kinds of tumors is underway. Clearly, there is an unmet clinical requirement for novel and/or more efficient combinatory therapies. Additionally, there is little information linking the incidence of TNBC in diabetic subjects.
In response to this emerging critical link between diabetes and triple-negative breast cancer (TNBC), the research team from WCM-Q, led by Dr. Dietrich Büsselberg, has set out to understand the elusive molecular mechanisms that contribute to diabetes-induced breast cancer and its progression in diabetic individuals. Through the support received from Qatar National Research Fund (QNRF) by a funded National Priorities Research Project (NPRP) project entitled “Anti-diabetic drugs in the treatment of breast cancer - identifying the molecular mechanism(s) and key biomarker(s)” (NPRP11S-1214-170101), the team outlines mechanism(s) attributed to this diabetes-breast cancer link, establishes potential therapeutic strategies, and identifies possible diagnostic/prognostic biomarkers.
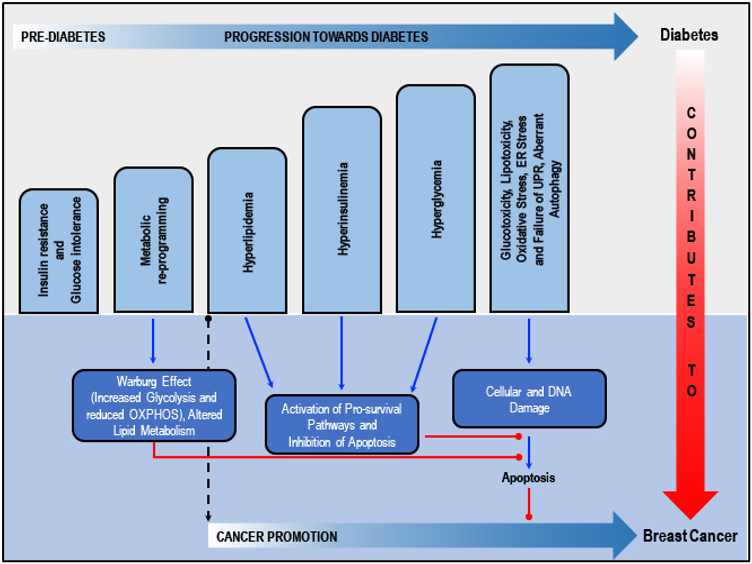
Shared factors in diabetes and breast cancer risk/progression: Insulin resistance, glucose intolerance and metabolic deregulation precede the development of hyperglycemia by years or even decades, ultimately leading to diabetes. Insulin resistance is compensated for by the secretion of supra-physiological amounts of insulin by the b-cells of the pancreas. Deterioration of the pancreatic b-cell function over time relates to insulin deficiency and ultimately hyperglycemia. Metabolic re-programming leads to altered carbohydrate and lipid metabolism in breast cancer cells. Diabetes associated hyperlipidemia, hyperinsulinemia and hyperglycemia activate several pro-survival pathways and inhibits apoptosis. Glucotoxicity-related oxidative stress, ER stress and failure of UPR and aberrant autophagy, cellular and DNA damage and failure of DNA repair mechanisms, plus inhibition of apoptosis, collaborate with the dysregulated metabolic and mitogenic mechanisms to initiate or promote cancer in breast cells. Adapted from our published article. doi: https://doi.org/10.1016/j.ctrv.2018.08.004
Drug screening (monotherapy and combination) for the treatment of BC
Natural substances: While some plant metabolites/derivatives induce the risk of cancers, many plant-derived active principles have gained attention as efficient anticancer agents against TNBCs, with fewer adverse side effects. At the Büsselberg lab, we focus on the possible oncogenic molecular pathways in TNBCs and how the purified plant-derived natural compounds specifically target and modulate the genes and/or proteins involved in these aberrant pathways to exhibit their anticancer potential. The team has produced peer-reviewed original and review articles related to the natural compounds for the treatment of cancer.
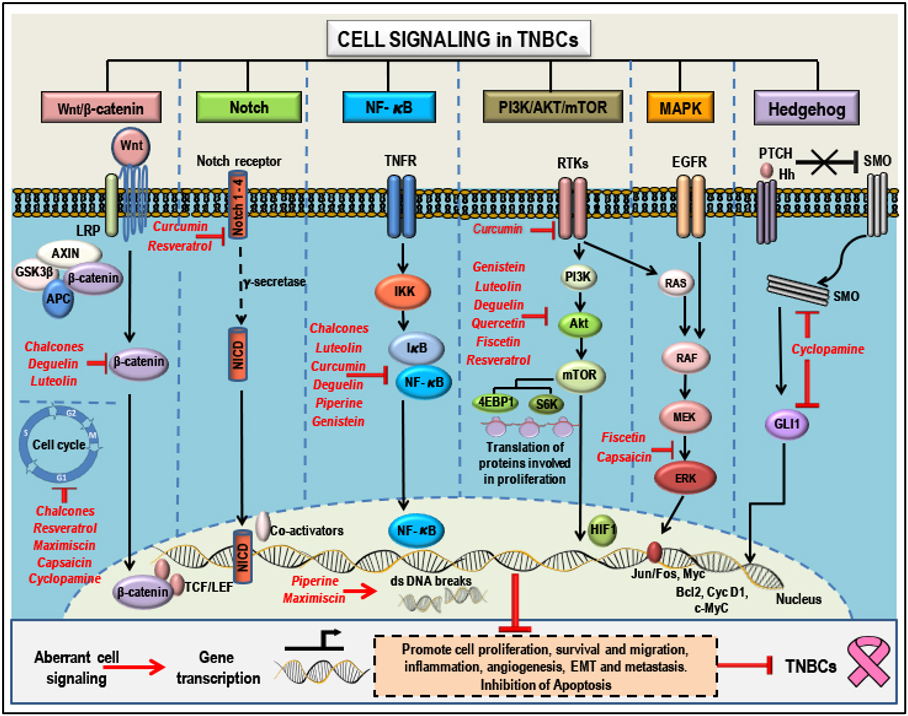
Natural compounds as anti-cancer agents in TNBCs: Mode(s) of Action: - Aberrant signaling pathways (Wnt/b-catenin, Notch, NF-kB, PI3K/AKT/mTOR, MAPK and Hedgehog) and pathway components that are targeted by natural compounds (highlighted in red). Phytochemicals have a broad range of action and a single anti-cancer phytochemical can target multiple pathways that determines the cell's fate. These compounds can suppress proliferation, growth and migration, cause cell cycle arrest, induce apoptosis, inhibit angiogenesis, suppress EMT and inhibit metastasis by targeting and modulating different pathway components and thereby regulating gene transcription and translation. This figure represents membrane, cytoplasmic and nuclear targets of the selected natural compounds which show potential anti-cancer properties in TNBCs. Adapted from our published article. doi: https://doi.org/10.3390/cancers10100346
We have linked the anticancer potential of plant-derived natural compounds (flavonoids, triptolide, luteolin, chalcones, piperine, deguelin, quercetin, rutin, fisetin, curcumin, resveratrol, and others) to their ability to target multiple dysregulated signaling pathways (such as the Wnt/β-catenin, Notch, NF-kB, PI3K/Akt/mammalian target of rapamycin (mTOR), mitogen-activated protein kinase (MAPK) and Hedgehog) leading to suppression of cell growth, proliferation, migration, inflammation, angiogenesis, epithelial-mesenchymal transition (EMT) and metastasis, and activation of apoptosis in breast cancers including TNBCs. Plant-derived compounds in combination with classical chemotherapeutic agents have shown to be more efficient in the treatment of TNBCs, possibly with lesser side effects.
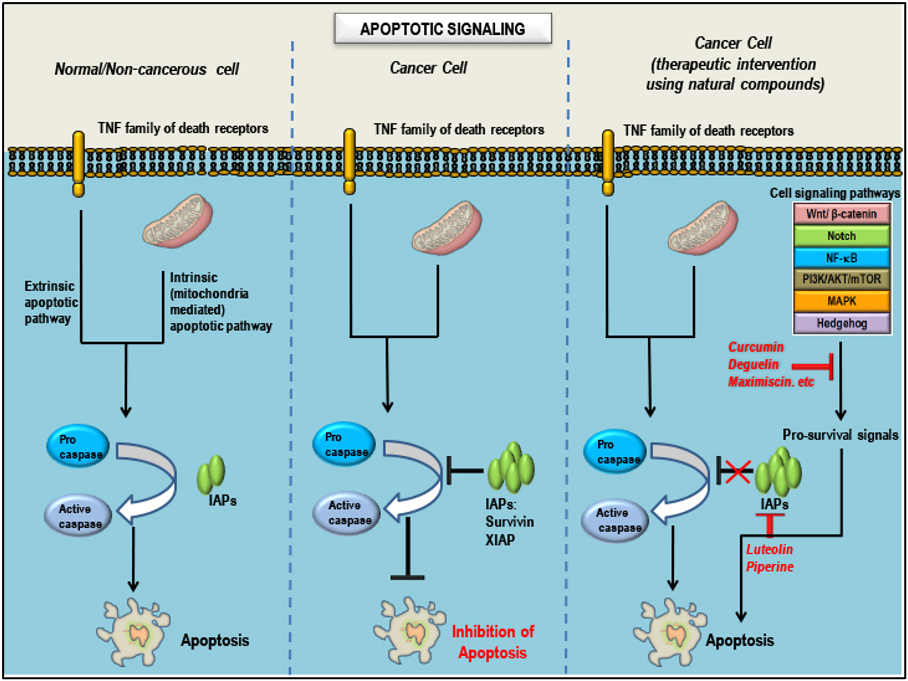
Natural compounds as pro-apoptotic agents in TNBCs: Mode(s) of Action: - Apoptotic signaling in three different cell conditions. Apoptosis is regulated by several proteins including caspases, proteins that promote apoptosis such as Smac/Diablo/Bax/Bad (positive regulators) and proteins that inhibit apoptosis such as survivin/Bcl2/Bcl-xL/IAPs/XIAP (negative regulators). In a non-cancerous cell, after completion of the normal life span, the cell undergoes apoptosis/programmed cell death. Inhibition of apoptosis is a hallmark of all cancers, which is characterized by abnormal expression patterns of different proteins involved in the apoptotic signaling which includes an increase in levels of negative regulators, decrease in the levels of positive regulators and inactivation of caspases. Treatment of cancers with natural compounds/phytochemicals that possess anti-cancer properties can block pro-survival signals from the aberrant signaling pathways involved in oncogenesis, decrease the levels of negative regulators, increase the levels of positive regulators and activate caspases, ultimately inducing apoptosis in cancer cells. Adapted from our published article. doi: https://doi.org/10.3390/cancers10100346
Anti-diabetic drugs in treatment of BC: Owing to the possible link between diabetes and cancer, our research group has been focusing on studying the anti-cancer properties of different groups of anti-diabetic drugs currently used for the treatment of diabetes. The team has realized the possible role of a widely prescribed anti-diabetic drug, metformin, in treating breast cancer. Metformin is easily synthesized, and, being well-tolerated and off-patent, can be made readily available to those in need. Reports suggest that metformin treatment in diabetic individuals significantly decreases the risk of developing breast cancer, inhibits cancer's progression and spread, and improves prognosis in affected individuals. Apart from its glucose-lowering effects, metformin possesses several anti-cancer properties that can be used in combination with other routinely used anti-cancer chemotherapeutic drugs and radiation for the effective treatment of breast cancer and several other cancers. The team intends to shed light on the molecular mechanisms involved in metformin's anti-cancer properties and identify cancer subjects who may benefit from metformin treatment alongside their cancer-directed therapy.
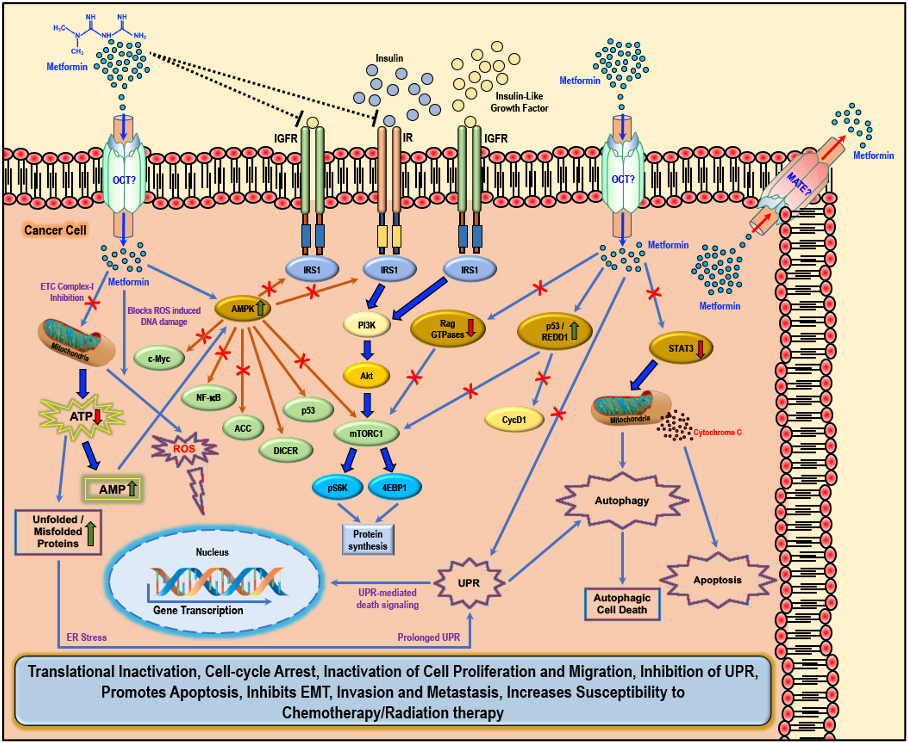
Cellular anti-cancer/anti-tumor effects of metformin: The hydrophilic and cationic metformin is transported into the cell via the organic cation transporters (OCT), which support the intracellular accumulation of metformin. The anti-proliferative activity of metformin in several cancers is at least in part attributed to its ability to reduce the levels of insulin/IGF1, which in turn inhibits the insulin/IGF1 mediated molecular pathways that support tumor initiation and progression. Metformin treatment directly activates AMPK and the ‘AMPK dependent’ effects include inhibition of c-Myc, NF-κB, and mammalian target of rapamycin-C1 (mTORC1) pathways and acetyl Co-A carboxylase (ACC)-dependent lipogenesis pathways while activating the p53 pathway and DICER-mediated miRNA synthesis. Metformin, albeit at high concentrations, is also known to inhibit the mitochondrial Complex 1 of the electron transport chain (ETC), thereby reducing ATP levels, which increases the AMP/ATP ratio further, leading to AMPK activation. A decrease in ATP/energy levels can also lead to mismanaged protein folding mechanisms, leading to the accumulation of unfolded or misfolded proteins, and prolonged unfolded protein response (UPR) (without rectification of endoplasmic stress) triggers apoptosis through multiple mechanisms, which include activation of UPR mediated apoptotic/death signaling and activation of autophagy and subsequent autophagic cell death. AMPK independent metformin treatment-associated anti-cancer effects are mediated by Rag GTPases, REDD1, and STAT3. Overall, metformin treatment in cancer cells causes translational inactivation, cell-cycle arrest, inactivation of cell proliferation and migration, inhibition of UPR, promotes apoptosis, inhibits EMT, invasion, and metastasis, and increases susceptibility to chemotherapy/radiation therapy. Adapted from our published article. doi: https://doi.org/10.3390/biom9120846
SARS-CoV-2 and Diabetes
Metformin intervention in SARS-CoV-2
The increasing prevalence of diabetes, the occurrence of diabetes-associated complications, and hence the proper management of blood glucose levels, has remained a persistent challenge for diabetic individuals and clinicians treating them. The management of blood glucose levels has become even more critical in the context of the COVID-19 pandemic, where the data, although limited, show two- to threefold higher intensive care hospital admissions and more than twice the mortality rate among diabetic COVID-19 patients when compared to their non-diabetic counterparts. While factors such as age, sex, ethnicity, and blood groups may be related to a poorer prognosis in diabetic COVID-19 patients, the existence of co-morbid conditions such as obesity, cardiovascular disease, and hypertension worsen the scenario. Furthermore, new-onset diabetes and severe metabolic complications are reported in COVID-19 patients.
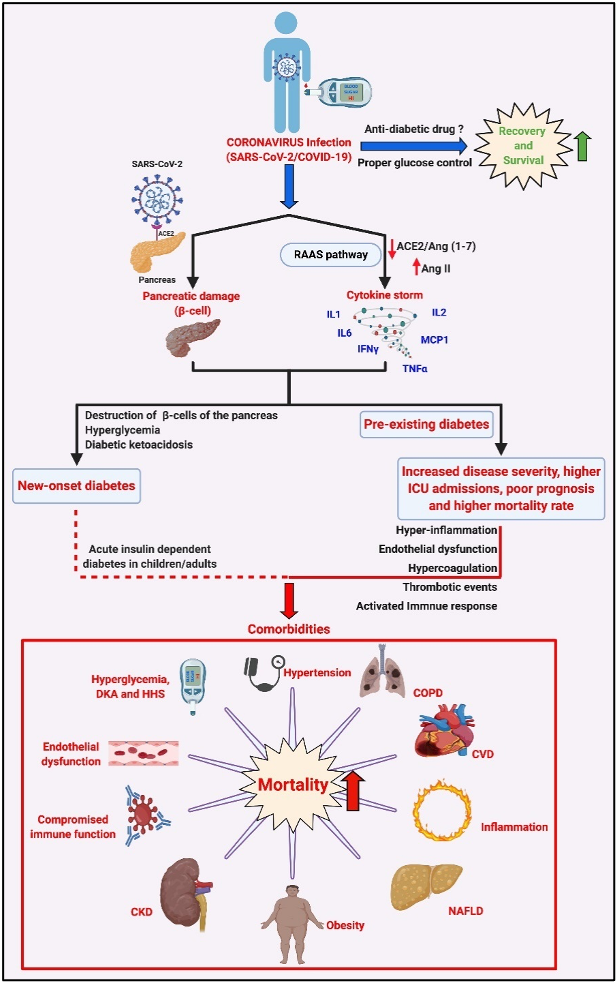
The relevance of diabetes, comorbidities, and management of blood glucose in the possible outcome of COVID-19 infection in diabetic patients: SARS-CoV-2 infection causes activation of RAAS that can result in a ‘cytokine storm’ via the AngII/AT1R axis resulting in the synthesis and secretion of pro-inflammatory cytokines/chemokines such as TNFa, IL1/2/6, interferon-γ (IFNg) and monocyte chemoattractant protein-1 (MCP1). SARS-CoV-2 infection in an individual with pre-existing diabetes, the existing pro-inflammatory status, and endothelial dysfunction, and the incidence of one or more comorbidities such as obesity, hypertension, CVD, NAFLD and CKD, and the hyperglycemia-induced DKA and HHS can lead to an increase in disease severity, higher rates of ICU admissions and may be responsible for the poor prognosis and higher mortality rates in diabetic COVID-19 patients. Interestingly, reports also suggest that SARS-CoV-2 infects the b-islets of the pancreas, causing b-cell damage and subsequent new-onset diabetes, severe hyperglycemia, and DKA in COVID-19 patients. Treatment using appropriate glucose-lowering agents and proper management of blood glucose levels aids recovery and survival among affected diabetic COVID-19 patients. Various aspects such as benefits, contra-indications, and limitations of using certain combinations of glucose-lowering agents and anti-viral treatments that could affect the disease's outcome in a diabetic COVID-19 patient must be carefully analyzed. CKD = Chronic Kidney Disease, COPD = Chronic Obstructive Pulmonary Disorder, CVD = Cardiovascular Disease, DKA = Diabetic Ketoacidosis, HHS = Hyperglycemic Hyperosmolar Syndrome, NAFLD = Non-Alcoholic Fatty Liver Disease. Adapted from our published article. doi: https://doi.org/10.1016/j.tim.2021.03.004
While there are several medications to manage blood glucose levels, researchers must tread carefully in this area, looking at various aspects such as benefits, contra-indications, and limitations of using certain combinations of glucose-lowering agents and anti-viral treatments that could affect the outcome of the disease in a diabetic COVID-19 patient. In this regard, metformin has gained global attention for its potential beneficial effects in the treatment of COVID-19 patients. Reports suggest that diabetic patients who were taking metformin had less severe symptoms and had lower rates of ICU admissions and death than those diabetic patients taking other anti-diabetic drugs to control their blood glucose levels. This indicated that metformin has a protective effect and could be used to supplement the treatment strategies we have in place for COVID-19. In our lab we want to focus on the potential mechanisms underlying the severity of the COVID-19 infection in diabetes while performing an in-depth assessment of the potential effects of the anti-diabetic drug metformin in diabetic COVID-19 patients. The potential multi-faceted benefits of metformin make it a viable candidate for drug re-purposing in the battle against COVD-19 in diabetic patients.
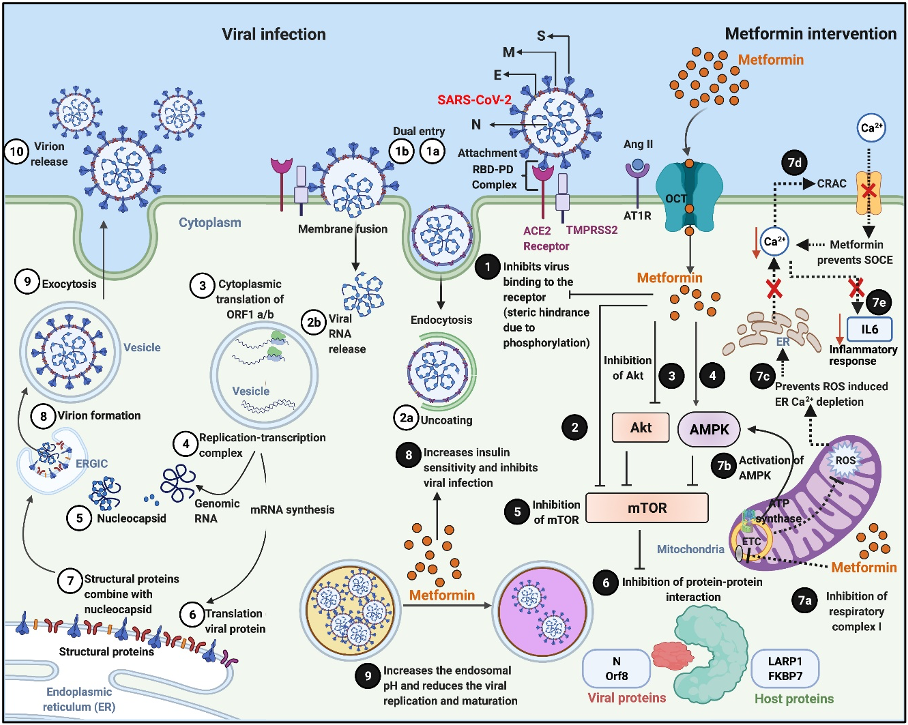
Viral pathogenesis and Metformin’s host- and virus-directed interventions in the attenuation of the disease process: Numerical indicators ①–⑩ illustrate the different stages of a typical viral infection, replication, transcription, protein translation, virion assembly, and release. Viral infection starts with binding of the spike [S] protein of the virus to the host receptor ACE2 to form the RBD–PD complex. This attachment is primed by another membrane protein called TMPRSS2 “①a.” “①b” indicates another form of viral entry through host membrane fusion. Viral entry by endocytosis is followed by uncoating “②a” and release of the viral RNA genome “②b,” which is translated into viral RNA polymerase proteins, resulting in the formation of ③ sub-genomic (−) RNAs then used as a template from subgenomic (+) mRNAs. After that, the following processes occur: the cytoplasmic viral RNA replication ④ and nucleocapsid [N] protein transcription ⑤ and subsequent translation ⑥ of structural viral proteins [S], membrane [M], and envelope [E] protein in the ER; followed by assembly of structural [S], [M], and [E] protein with the [N] protein viral genome RNA complex ⑦ and mature virion assembly ⑧ in the ERGIC. The assembled virions bud off from the Golgi vesicle ⑨ and are released ⑩ by exocytosis. Numerical indicators ❶–❾ illustrate the potential sites of virus–host-directed action of Metformin. ❶ Metformin can potentially inhibit the virus’s attachment to the host plasma membrane by blocking the virus from binding to the host receptor ACE2. Metformin directly ❷, indirectly via Akt inhibition ❸ or via AMPK activation ❹ inhibits mTOR activity ❺ resulting in the suppression of virus–host protein interaction ❻. Inhibition of respiratory complex I by Metformin “❼a” activates AMPK “❼b” and results in inhibiting the mTOR signaling. Metformin also inhibits mitochondrial generation of ROS “❼c,” which subsequently prevents ROS-induced ER calcium (Ca2+) depletion and suppresses the activation of CRAC channel “❼d,” preventing SOCE ultimately preventing a rise in intracellular Ca2+ and subsequent release of the inflammatory mediator IL-6 “❼e,” which is often associated with COVID-19 and mediates thrombosis. Metformin increases insulin sensitivity ❽ and inhibits viral infections, modifies endosomal pH, and reduces viral ❾ replication and maturation. ACE2, angiotensin converting enzyme 2; COVID-19, Coronavirus Disease 2019; CRAC, Ca2+ release-activated Ca2+; ER, endoplasmic reticulum; ERGIC, ER–Golgi intermediate compartment; IL, interleukin; PD, peptidase domain; RBD, receptor-binding domain; ROS, reactive oxygen species; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; SOCE, store-operated calcium entry. Adapted from our published article. doi: https://doi.org/10.1371/journal.ppat.1009634
Past Projects of Interest
- Pain modulation and Natural Substances
- Respiratory Rhythm Generation
- Metal neurotoxicity