Overview
Research in the Triggle Laboratory focuses on the role of the endothelium in the regulation of vascular tone in normal and pathophysiological states. The current focus is on studies directed towards understanding the cellular basis for diabetes-related vascular disease and optimising therapeutic and regenerative interventions that can protect the endothelium and repair and restore normal endothelial function and prevent disease progression. Also see entry for Dr. Isra Marei, a member of Dr. Triggle’s research team.
An overview of the contributions of Dr. Triggle’s research to our understanding of smooth muscle and cardiovascular physiology and pharmacology is provided by Google Scholar revealing >9500 citations and an h-index of 54:
https://scholar.google.com/citations?user=3b5nJfAAAAAJ&hl=en
Dr. Triggle is involved in international research collaborations that include Professor Morley Hollenberg, The University of Calgary Cumming School of Medicine, with whose research group we have several jointly authored publications.
Progress in our knowledge of endothelial function and dysfunction has been summarized in the following review published in 2020:
Triggle CR, Ding H, Marei I, Anderson TJ, Hollenberg MD. (2020). Why the endothelium? The endothelium as a target to reduce diabetes-related associated vascular disease. Can J Physiol Pharmacol. 2020 Jul; 98(7):415-430. doi: 10.1139/cjpp-2019-0677. PMID: 32150686
The widely used anti-diabetic drug metformin and first choice agent for most people with type 2 diabetes has been the focus of our research for a number of years and a publication in Molecular Pharmacology provides evidence that metformin protects the endothelium against hyperglycaemia via targeting the orphan nuclear receptor, Nr4a1. These findings open up a new area of research and the understanding of how to both prevent and treat vascular disease.
Vivek Krishna Pulakazhi Venu, Mahmoud Saifeddine, Koichiro Mihara, Muniba Faiza, Evgueni Gorobets, Andrew J. Flewelling, Darren J. Derksen, Simon A. Hirota, Isra Marei, Dana Al-Majid, Majid Motahhary, Hong Ding, Chris R. Triggle and Morley D. Hollenberg. (2021) ‘Metformin prevents hyperglycaemia-associated, oxidative stress-induced vascular endothelial dysfunction: essential role for the orphan nuclear receptor, Nr4a1 (Nur77)’. Molecular Pharmacology. Aug 27:MOLPHARM-AR-2020-000148. doi: 10.1124/molpharm.120.000148. PMID: 34452975.
The schematic below is from our 2021 publication in Frontiers in Endocrinology:
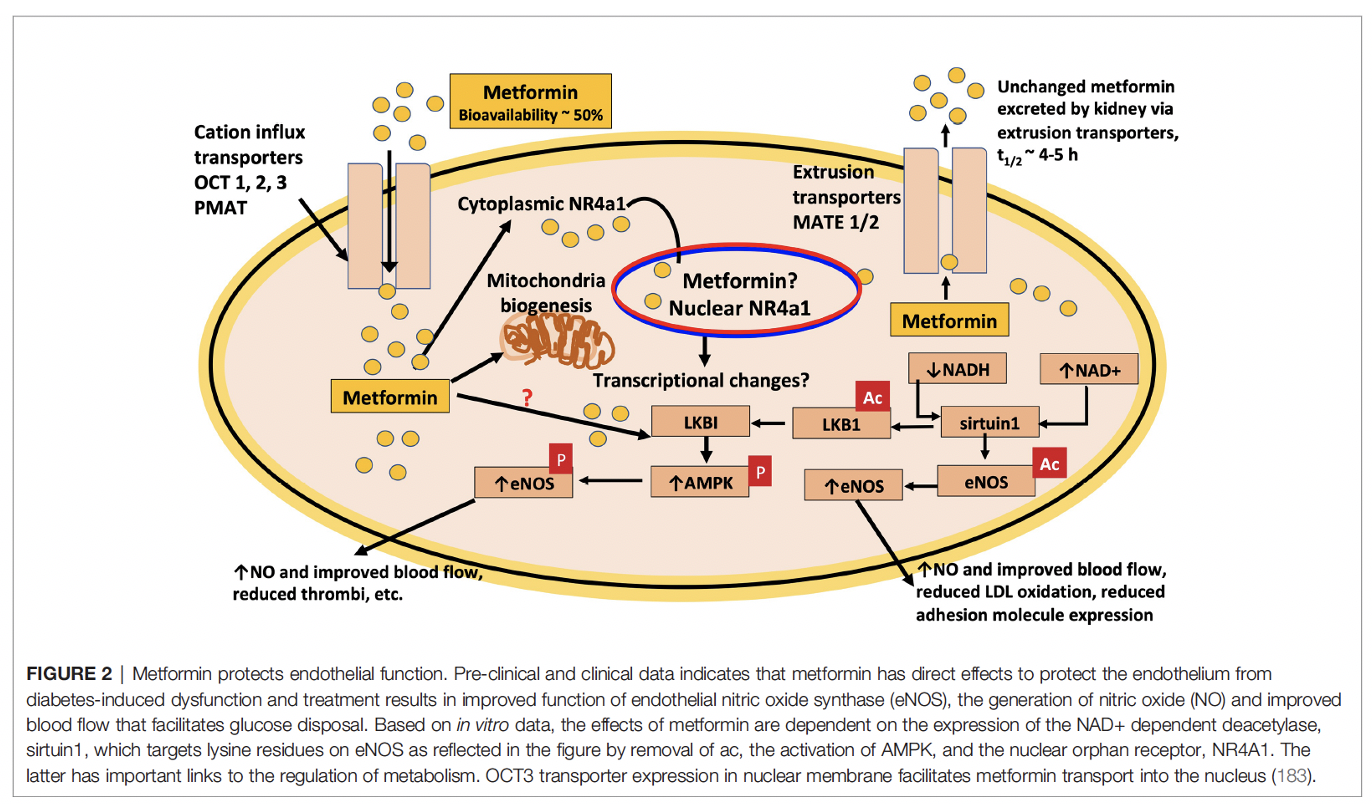
Metformin has also been the center of attention as a drug that can be re-purposed for a multitude of diseases including as an anti-aging drug, to treat neurodegenerative diseases, for cancer, and also for COVID-19. We are actively critically evaluating the literature with the objective to provide an informed opinion that is based on reproducible medical evidence. The following publications address the claims that metformin can be re-purposed as a drug to treat cancer as well as be used as an anti-aging drug:
Samuel, S.M., Varghese, E., Kubatka, P., Triggle, C.R., Busselberg, D. 2019. Metformin: The Answer to Cancer in a Flower? Current Knowledge and Future Prospects of Metformin as an Anti-Cancer Agent in Breast Cancer. Biomolecules 9(12):846. doi: 10.3390/biom9120846.
Ibrahim Mohammed, Hong Ding, Morley D. Hollenberg, and Chris R. Triggle. (2021) ‘A review of the effects of metformin as a putative anti-aging drug that enhances healthspan and extends lifespan’. Frontiers in Endocrinology, Aug 5;12:718942. doi: 10.3389/fendo.2021.718942. eCollection 2021. PMID: 34421827
Dr. Triggle is also active in the ethics of research with a particular emphasis on promoting open access as well as addressing issues such as bias in peer review, citation manipulation and fraud. The following publications provide an indication of the scope of these interests:
Triggle CR, Triggle DJ. (2017). From Gutenberg to Open Science: An Unfulfilled Odyssey. Drug Development Research 2017;78(1):3-23. doi: 10.1002/ddr.21369.
Chris R. Triggle, Ross MacDonald, David J Triggle, Donald Grierson. (2021). Requiem for impact factors and high publication charges. Accountability in Research: Policies and Quality Assurance. Published on line March 25th 2021. doi: 10.1080/08989621.2021.1909481