Quality: Definitions and Challenges
The definition of quality in health care has evolved over the last century. In 1933 in “The fundamentals of good medical care” Lee and Jones defined good medical care as “the kind of medicine practiced and taught by the recognized leaders of the medical profession” and they go on further defining this by providing eight “articles of faith”, many of which are still relevant today 1. A more modern definition was provided by the committee on quality and healthcare in America, which stated that healthcare should be safe, effective, patient-centered, timely, efficient, and equitable 2. However, even with existing general definitions of quality in healthcare, there is still a fairly wide discrepancy in what individuals may understand as quality. This is often based on very different objectives which can have a wide discrepancy when you compare patients, physicians, and payers.
In general, we differentiate quality assurance from (continuous) quality improvement, and while we often look at outcomes as the main measure of quality, there are many other measures that are related to the structure as well as the process of how the care is being delivered.
There has been a notable increase in the interest on quality in healthcare over the last two decades. Publications searchable on PubMed and focusing on congenital heart disease and quality have increased by almost 10-fold over the last 25 years, from 54 publications between 1991-1995 to 541 publications from 2011-2015. While this in its own does not guarantee any improvement in quality, an increasing awareness is a crucial ingredient for a successful quality improvement process. Verghese and colleagues from Boston reported in 2012 on the effect of a radiation-monitoring program in a large volume pediatric cath lab, and found that just by introducing the radiation threshold monitoring and notification policy, there was a statistically significant decrease in the radiation dose 3.
The quality assurance and quality improvement process in the congenital cardiac catheterization laboratory was plagued with a variety of problems and only started to improve over the last two decades. This included the lack of an agreed nomenclature for procedure types, the lack of agreed definitions for complications or severity classifications 4, 5 , the lack of active registries, as well as the lack of adjustment methods for case mix and complexity. Outcomes were usually reported as single center outcomes and were very procedure specific. As such, quoted incidences of complications were almost impossible to compare and what was considered a complication (or adverse event) was to some degree subjective, depending on the authors (institution) of the relevant paper.
The first registry reporting on outcomes of congenital cardiac catheterization was the VACA registry in the 1990s, which essentially collected outcomes in the very early experience of balloon angioplasty and valvuloplasty 6, 7 . This was then followed by FDA sponsored device trials, and subsequently investigator organized registries such as CCISC, MAGIC, and C3PO, which started to come to the forefront in the early 2000s. The latest step was the introduction of large societal registries such as IMPACT, which has been the largest registry of its kind for the last 5 years 8, 9 .
Quality assurance and improvement in the congenital cath lab faces a variety of challenges, which include the heterogeneity of patients, with a wide variation in case complexity and acuity. In addition, standard outcome parameters such as mortality that are important markers of outcome for our surgical colleagues are less useful in the congenital cardiac catheterization laboratory due to their low incidence. Furthermore, some well-established outcome parameters may not necessarily reflect the outcome of the catheterization procedure that is being performed, but instead reflect a preceding cardiac surgery or other events. As an example, the 30-day mortality was originally utilized and included in the outcome metrics of the IMPACT registry. However, subsequent data has shown that only a small portion of deaths occurring within 30 days were actually attributable to the cath procedure itself 10. Therefore, while 30-day mortality may be helpful defining the overall institutional outcomes (including cardiac surgery), it is less helpful when trying to improve the core interventional catheterization program. Eventually this was recognized and therefore version two of the IMPACT registry does not include 30-day mortality (better: mortality per episode of care) in the core outcome metrics.
Other important limitations of assessing quality in the congenital catheterization laboratory relate to the lack of evidence based established criteria of procedural efficacy. Of course, adverse events are an important outcome parameter when assessing the quality of the work performed. However, adverse events cannot be looked at in isolation, but instead require careful evaluation of the procedural efficacy. If a procedure does not achieve the intended outcome, one could argue that this in fact should be classified as an adverse event on its own.
Comparison of Outcomes
Realizing the shortcomings of the existing outcome assessments for congenital cardiac catheterization, the congenital cardiac catheterization project on outcomes (C3PO) was formed under the leadership of Lisa Bergersen from Boston Children’s Hospital 11. C3PO started a multi institutional registry with initially six and later eight participating centers. The purpose of the registry was to assess and compare outcomes in pediatric and congenital cardiac catheterization and to develop a risk adjustment model for these procedures. Prospective data collection started in February 2007, using web-based data entry. The severity of adverse events was classified into 5 levels, with higher severity adverse events being classified as level 3-5, and life threatening adverse events as level 4-5 (Figure 1).
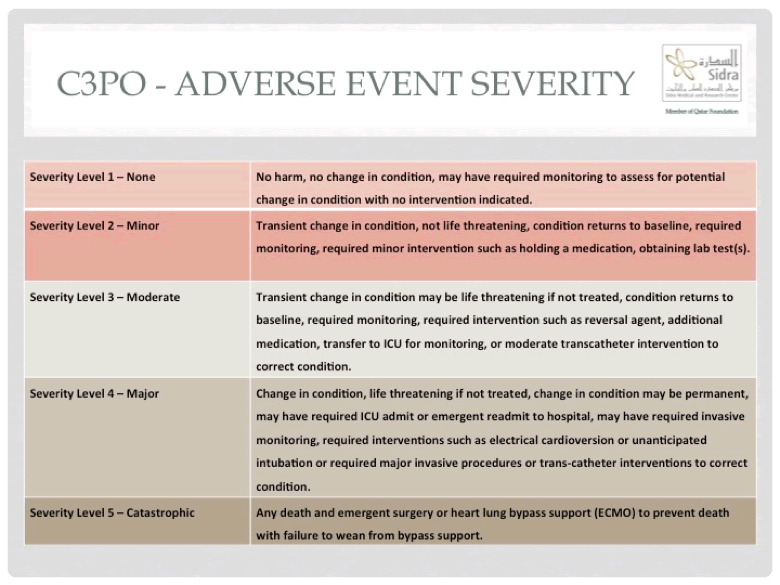
Figure 1
The data originating from C3PO for the first time provided operators with more accurate information on the incidence of adverse events. The overall incidence of adverse events was 19% for interventional and 10% for diagnostic cases, with the incidence of higher severity adverse events being 9% for interventional and 5% for diagnostic cases
11. Level 4 adverse events were seen in 1.4% of cases, with death occuring in 0.2% of cases
12.
The main purpose though of C3PO was to provide a risk adjustment model. To achieve this, procedures were initially classified into six different categories, using a consensus based approach, and then the subsequently gathered data was used to validate (and modify) the chosen categories. This resulted in four distinct procedure type risk categories 13. While this allowed adjustment for various risks associated with the procedure itself, the model did not take account of the inherent risks associated with any specific patient. To account for this, a variety of hemodynamic variables were chosen in a consensus based fashion and then different thresholds and combination of variables evaluated using the empirically gathered data. The final grup of hemodynamic variables (Figure 2), combined with procedure type risk group and age of the patient above or below 1-year formed the catheterization for congenital heart disease adjustement for risk method (CHARM) 14. The standardized adverse event ratio is calculated based on the CHARM model and divides the occuring by the expected number of adverse events based on the institutional or operator-specific patient and procedure profile, and was the first accepted quality metric by the National Quality forum for congenital cardiac catheterizarion procedures.
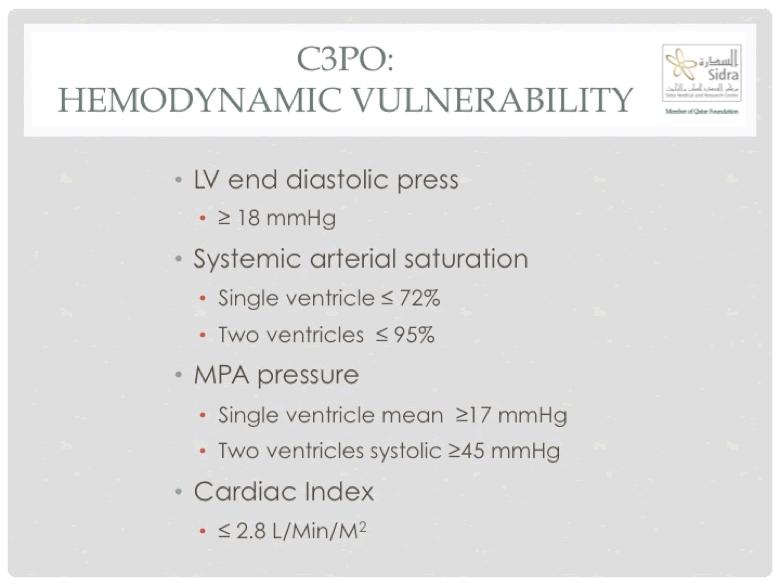
Figure 2
Following CHARM, a larger data set subsequently gathered by the IMPACT registry was used to validate and further modifiy the risk adjustment model. It uses further sub categories for age as well as adding the presence of renal insufficieny or single ventricle physiology as additional risk factors 15. IMPACT is expected to use this risk adjustment model to compare adverse event rates between institutions, and that data will have considerable importance in areas such as cath lab accreditation by the joint venture of SCAI and ACCF Accreditation for Cardiovascular Excellence (ACE).
The data gathered from the C3PO registry allowed to further look at a variety of specific procedure types, such as pulmonary or aortic valvuloplasty, pulmonary artery rehabilitation, hybrid procedures, and many more to identify additional procedure specific independent risk factors, but more importamtly to add efficacy parameters for specific proceure types 16-20.
In addition to procedure specific data, the data captured by C3PO was used to evaluate the impact of operator experience om the incidence of procedural adverse events, identifying operators with less than 5 years practice to have higher risk adjusted adverse event rates, in particular for preventable or possibly preventable adverse events (Figure 3) 21.
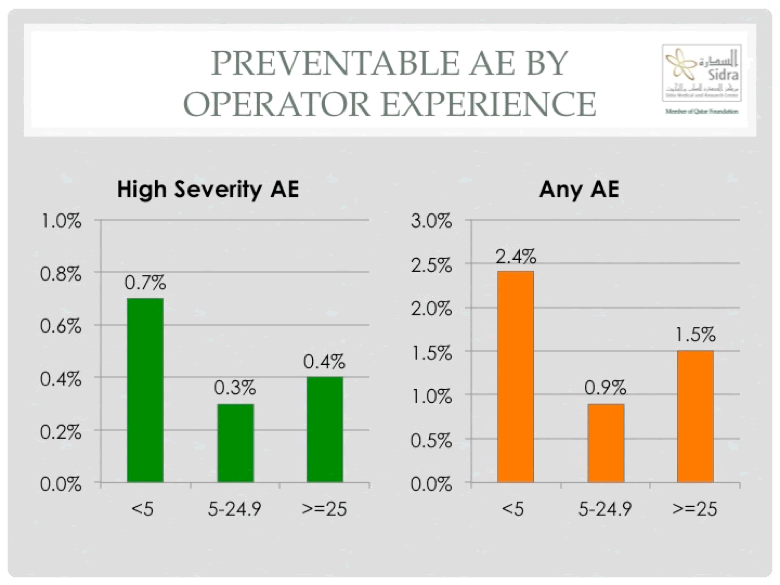
Figure 3
Quality Assurance and Continuous Quality Improvement
Individual centers are often faced with the difficulty of establishing a flourishing assurance and continuous quality improvement program in the congenital cardiac catheterization laboratory. Cornerstones of those activities are local quality assurance, local (continuous) quality improvement, participation in medium-sized research registries and associated quality improvement efforts, participation in larger scale societal registries, as well as cath lab accreditation.
Frequently, when trying to establish an active QA/QA program resistance may be encountered from colleagues and members of staff. In that context it is important to emphasize that the goal being not to find those who are underachieving (as a punitive measure), but instead allow individuals and programs to identify areas of potential improvement and provide methods to achieve that improvement. Once this message comes across loud and clearly, it will be much easier to get buy-in from colleagues and staff, which is crucial for the success of the program. The QI/QA process also has important implications for cath lab accreditation, as the ACE standards documents for congenital heart disease recommends a member of staff to be named to be responsible for overseeing the QI/QA process in the cath lab.
Local quality assurance includes elements such as documenting compliance with existing standards, key performance measures, regular cath quality conferences, and institutional event monitoring. Having consistent and accurate data capture with the ability to extract data quickly and easily is crucial to support those activities. The easier it is to obtain the data one needs, the more likely the data will be used for regular QA and QI purposes. As such, investment in the appropriate database solution that can support the local data needs is important.
Local quality improvement in the congenital catheterization laboratory offers ample of opportunity for all levels of staff to become actively engaged in the process. This work can be very rewarding, especially if staff sees that with very little effort, it is possible to achieve a significant reduction in potential harm to the patient (Figure 4) 22. Examples of quality improvement projects that can easily be established locally include a reduction in radiation exposure, completeness of handoffs, as well as prevention of skin-related injuries.
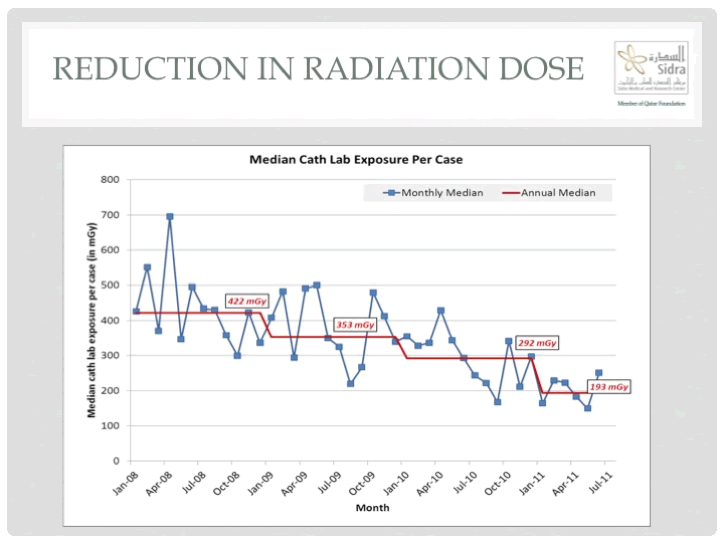
Figure 4
Quality assurance and continuous quality improvement go hand in hand with registry participation, which form the cornerstone to evaluate outcomes in the congenital cath lab. Lucas Kappenberger (at Heart Rhythm Society Policy Conference, Washington, DC, 2005) exquisitely described the importance of registries by stating that “Science tells us what we can do; Guidelines what we should do; & Registries what we are actually doing”. The problem of institutional data are small numbers and a large variation in complexity and acuity, which makes it difficult to analyze as a single center whether the outcomes are as expected. In contrast, registries can provide benchmark data as well as the possibility for risk stratification, while also supplementing the QA and QI process (including MOC 4 credit from many registries) 23.
Not every registry is equally suitable for each center, so a selection has to be made by each center depending on individual goals. Medium sized investigator-organized research registries may have distinct advantages for the local QI and QA process, by providing better control and access to the data and a higher degree of flexibility to change compared to larger societal registries such as IMPACT. They are also much mores suitable to advance specific research projects with a smaller administrative overhead. In addition, many of those registries provide easily accessible tools for ad-hoc data reports and data analysis, that can be used whenever needed to supplement the local QI and QA process. As a disadvantage though, participation in these registries can be somewhat selective and the number of participating sites may be fairly limited, thereby leading longer required periods of data collection to achieve the necessary power to report on specific outcomes. Furthermore, many payers and organizations have adopted outcome metrics of the IMPACT registry (such as ACE accreditation), which makes it very helpful to also participate in IMPACT. In the future, participation in IMPACT may potentially become mandated for reimbursement of some procedures, as has been seen with longer established adult NCDR registries. IMPACT also uses a well-established infrastructure of the National Cardiovascular Data Registry and as such is very robust. However, this also has some disadvantage in being slow to change and limiting ad-hoc data access.
Despite the multiple advantages of registry participation, there are also some potential problems related to data capture in the cath lab in general, and more specifically to registry participation. Data entry and validation is time consuming and ensuring long-term data accessibility can be difficult when participating in registries. Furthermore, institutions may have very little control on how data is being used once it is submitted to a registry. Ultimately though, the goal of any registry participation is to improve overall quality and outcomes, and as such it is probably the most important tool institutions have to support the QI and QA process.
Summary
As a summary, quality has evolved as the most important focus for medicine in general, and the congenital catheterization laboratory in particular over the last decade. Comparison of outcomes is crucial for the QI and QA process, and registry participation is one of the most important tools to support these efforts.
References:
- Lee RI and Jones LW. The fundamentals of Medical care: An Outcline of the Fundamentals of Good Medicine and an Estimate of the Service Required to Supply Medical needs of the United States. Committee on the Costs of Medical Care no. 22. University of Chicago Press. 1933.
- Crossing the Quality Chasm: A New Health System for the 21st Century. Institute of Medicine (US) Committee on Quality of Health Care in America. Washington (DC): National Academic Press (US). 2001.
- Verghese GR, McElhinney DB, Strauss KJ and Bergersen L. Characterization of radiation exposure and effect of a radiation monitoring policy in a large volume pediatric cardiac catheterization lab. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2012;79:294-301.
- Bergersen L, Everett AD, Giroud JM, Martin GR, Franklin RC, Beland MJ, Krogmann ON, Aiello VD, Colan SD, Elliott MJ, Gaynor JW, Kurosawa H, Maruszewski B, Stellin G, Tchervenkov CI, Walters HL, 3rd, Weinberg P and Jacobs JP. Report from The International Society for Nomenclature of Paediatric and Congenital Heart Disease: cardiovascular catheterisation for congenital and paediatric cardiac disease (Part 1 - Procedural nomenclature). Cardiol Young. 2011;21:252-9.
- Bergersen L, Giroud JM, Jacobs JP, Franklin RC, Beland MJ, Krogmann ON, Aiello VD, Colan SD, Elliott MJ, Gaynor JW, Kurosawa H, Maruszewski B, Stellin G, Tchervenkov CI, Walters HL, Weinberg P and Everett AD. Report from The International Society for Nomenclature of Paediatric and Congenital Heart Disease: cardiovascular catheterisation for congenital and paediatric cardiac disease (Part 2 - Nomenclature of complications associated with interventional cardiology). Cardiol Young. 2011;21:260-5.
- Allen HD and Mullins CE. Results of the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol. 1990;65:772-4.
- Stanger P, Cassidy SC, Girod DA, Kan JS, Lababidi Z and Shapiro SR. Balloon pulmonary valvuloplasty: results of the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol. 1990;65:775-83.
- Holzer R, Beekman R, Benson L, Bergersen L, Jayaram N, Jenkins K, Kennedy K, Moore J, Ringel R, Rome J, Vincent R and Martin GR. Characteristics and safety of interventions and procedures performed during catheterisation of patients with congenital heart disease: early report from the national cardiovascular data registry. Cardiol Young. 2015:1-11.
- Martin GR, Beekman RH, Ing FF, Jenkins KJ, McKay CR, Moore JW, Ringel RE, Rome JJ, Ruiz CE and Vincent RN. The IMPACT registry: IMproving Pediatric and Adult Congenital Treatments. Seminars in thoracic and cardiovascular surgery Pediatric cardiac surgery annual. 2010;13:20-5.
- Backes CH, Bergersen L, Rome JJ, Batlivala SP, Glatz AC, Ovunc B, David S, Rivera BK, Haque U, Kollins K, Yin H and Holzer RJ. Quality metrics in cardiac catheterization for congenital heart disease: utility of 30-day mortality. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2015;85:104-10.
- Bergersen L, Marshall A, Gauvreau K, Beekman R, Hirsch R, Foerster S, Balzer D, Vincent J, Hellenbrand W, Holzer R, Cheatham J, Moore J, Lock J and Jenkins K. Adverse event rates in congenital cardiac catheterization - a multi-center experience. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2010;75:389-400.
- Lin CH, Hegde S, Marshall AC, Porras D, Gauvreau K, Balzer DT, Beekman RH, 3rd, Torres A, Vincent JA, Moore JW, Holzer R, Armsby L and Bergersen L. Incidence and management of life-threatening adverse events during cardiac catheterization for congenital heart disease. Pediatr Cardiol. 2014;35:140-8.
- Bergersen L, Gauvreau K, Marshall A, Kreutzer J, Beekman R, Hirsch R, Foerster S, Balzer D, Vincent J, Hellenbrand W, Holzer R, Cheatham J, Moore J, Lock J and Jenkins K. Procedure-type risk categories for pediatric and congenital cardiac catheterization. Circulation Cardiovascular interventions. 2011;4:188-94.
- Bergersen L, Gauvreau K, Foerster SR, Marshall AC, McElhinney DB, Beekman RH, 3rd, Hirsch R, Kreutzer J, Balzer D, Vincent J, Hellenbrand WE, Holzer R, Cheatham JP, Moore JW, Burch G, Armsby L, Lock JE and Jenkins KJ. Catheterization for Congenital Heart Disease Adjustment for Risk Method (CHARM). JACC Cardiovasc Interv. 2011;4:1037-46.
- Jayaram N, Beekman RH, 3rd, Benson L, Holzer R, Jenkins K, Kennedy KF, Martin GR, Moore JW, Ringel R, Rome J, Spertus JA, Vincent R and Bergersen L. Adjusting for Risk Associated With Pediatric and Congenital Cardiac Catheterization: A Report From the NCDR IMPACT Registry. Circulation. 2015;132:1863-70.
- Torres A, Vincent JA, Everett A, Lim S, Foerster SR, Marshall AC, Beekman RH, 3rd, Murphy J, Trucco SM, Gauvreau K, Holzer R, Bergersen L and Porras D. Balloon valvuloplasty for congenital aortic stenosis: Multi-center safety and efficacy outcome assessment. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2015;86:808-20.
- El-Said HG, Bratincsak A, Foerster SR, Murphy JJ, Vincent J, Holzer R, Porras D, Moore J and Bergersen L. Safety of percutaneous patent ductus arteriosus closure: an unselected multicenter population experience. Journal of the American Heart Association. 2013;2:e000424.
- Holzer RJ, Gauvreau K, Kreutzer J, Trucco SM, Torres A, Shahanavaz S and Bergersen L. Safety and efficacy of balloon pulmonary valvuloplasty: a multicenter experience. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2012;80:663-72.
- Holzer RJ, Gauvreau K, Kreutzer J, Leahy R, Murphy J, Lock JE, Cheatham JP and Bergersen L. Balloon angioplasty and stenting of branch pulmonary arteries: adverse events and procedural characteristics: results of a multi-institutional registry. Circulation Cardiovascular interventions. 2011;4:287-96.
- Holzer R, Marshall A, Kreutzer J, Hirsch R, Chisolm J, Hill S, Galantowicz M, Phillips A, Cheatham J and Bergerson L. Hybrid procedures: adverse events and procedural characteristics--results of a multi-institutional registry. Congenital heart disease. 2010;5:233-42.
- Holzer RJ, Gauvreau K, Kreutzer J, Moore JW, McElhinney DB and Bergersen L. Relationship between procedural adverse events associated with cardiac catheterization for congenital heart disease and operator factors: results of a multi-institutional registry (C3PO). Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2013;82:463-73.
- Sawdy JM, Kempton TM, Olshove V, Gocha M, Chisolm JL, Hill SL, Kirk A, Cheatham JP and Holzer RJ. Use of a dose-dependent follow-up protocol and mechanisms to reduce patients and staff radiation exposure in congenital and structural interventions. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2011;78:136-42.
- Ghelani SJ, Glatz AC, David S, Leahy R, Hirsch R, Armsby LB, Trucco SM, Holzer RJ and Bergersen L. Radiation dose benchmarks during cardiac catheterization for congenital heart disease in the United States. JACC Cardiovasc Interv. 2014;7:1060-9.
Written for April 2016 by
Ralf J. Holzer, MD, MSc, FSCAI
Professor of Clinical Pediatrics
Weill Cornell Medicine-Qatar
Director, Cardiac Catheterization & Interventional Therapy
Division Chief Cardiology (Acting)
Sidra Medical and Research Center